Understanding the mRNA Flu Vaccine: What You Need to Know

A New Approach to Flu Prevention
Every year, influenza (the flu) returns with new strains that can cause serious illness, especially in adults, older populations, and those with underlying health conditions. While annual flu vaccines remain the best defense against influenza, their protection can vary depending on how closely they match circulating viruses.
That’s why scientists are developing a new flu vaccine using mRNA technology—an approach that could make flu shots faster to update, more effective, and better suited for changing flu seasons.
Here’s what you need to know.
What is an mRNA Flu Vaccine?
At its core, mRNA vaccines work by delivering a set of instructions, or messenger ribonucleic acid (mRNA), to your ribosomes (protein-making structure cells). The ribosomes then use those instructions to allow your body to create, recognize, and then fight viruses. Similarly to other vaccines, the goal is to train your body to recognize and create the tools to fight harmful diseases (like COVID, the Flu, etc.). Instead of using a weakened or inactivated flu virus like the traditional vaccine, the mRNA flu vaccine uses messenger RNA to teach your immune system how to recognize and fight off influenza. (source: Cleveland Clinic: mRNA Vaccines)
Think of mRNA as a recipe card: once your cells “read” the recipe and create a harmless piece of the flu virus (called an antigen), your immune system practices fighting it. If you’re later exposed to the real flu virus, your body is prepared to respond quickly.
Importantly, the mRNA doesn’t stay in your system—it naturally breaks down within a few days after delivering its instructions. This makes it a clean, efficient way to help your body build protection.
Why Develop a New Flu Vaccine?
The flu isn’t a static virus; it evolves every year. That’s why flu shots are reformulated annually, and even then, effectiveness can vary. The CDC has been conducting studies each flu season to determine the efficacy of flu vaccines, in which most years the rate of vaccine effectiveness falls between 40%-60%. (source: CDC Seasonal Flu Vaccine Effectiveness Studies)
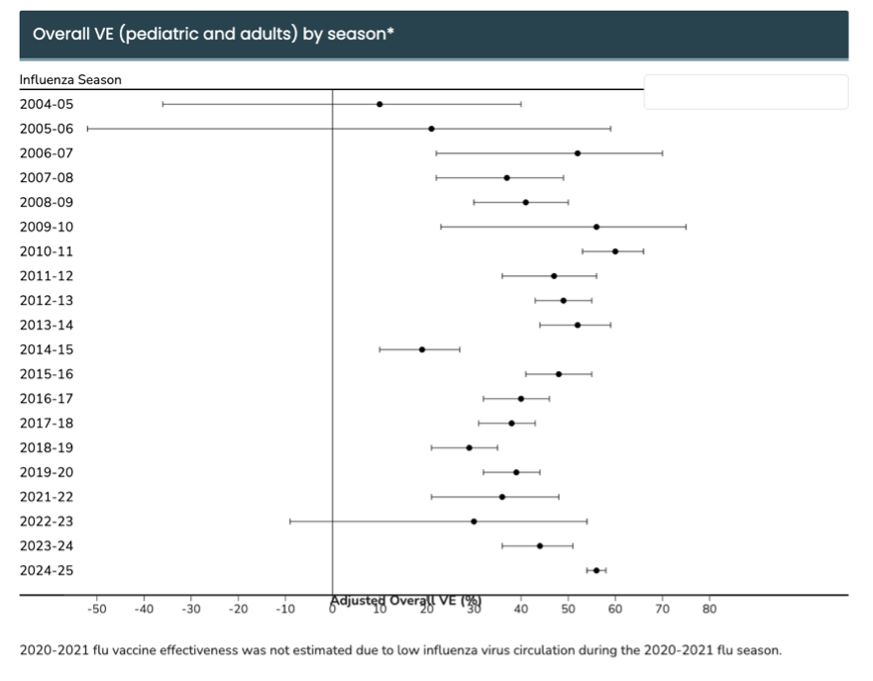
Flu vaccine research has long aimed to solve this problem. By using mRNA, scientists hope to create vaccines that:
- Can be updated more quickly when flu strains shift.
- Offer broader immune protection across multiple strains.
- Potentially improve outcomes for adults and older adults who face the highest risks.
This is especially important as we prepare for flu season 2025 and beyond, as there are currently three main flu viruses. To read more on how flu vaccine strains are chosen each year and the various strains of flu, refer to Dr. Angela Rasmussen and Candace Lamb’s article on VIDO.
The Benefits of an mRNA Flu Vaccine
While research is ongoing, potential advantages of an mRNA flu vaccine include:
- Faster Updates: mRNA technology allows scientists to adjust the vaccine more quickly to match changing flu strains.
- Stronger Immune Response: Early studies suggest mRNA vaccines can trigger a more robust response, which may improve flu vaccine effectiveness.
- Adaptability for Adults: Because older adults often respond less strongly to traditional flu vaccines, an mRNA option could improve influenza prevention in this population.
- Scalability: In the event of a severe flu season or pandemic, mRNA vaccines may be produced and distributed faster than traditional options.
How Clinical Trials Make New Flu Vaccines Possible
Before any new flu vaccine becomes widely available, it must go through rigorous flu vaccine studies to test safety and effectiveness. Clinical trials are the only way to answer critical questions surrounding the effectiveness and safety of new mRNA vaccines. Every vaccine you’ve ever received—including the seasonal flu shot—was once tested in a trial just like this.
What This Means for You
For many, the flu is more than an inconvenience. It can cause serious complications like pneumonia, hospitalization, or worse—especially in older adults and those with chronic health conditions.
That’s why ongoing flu vaccine research is so important. By joining a clinical trial, participants not only gain early access to cutting-edge vaccine technology but also play a direct role in shaping the future of flu prevention.
At Health Research of Hampton Roads (HRHR), we will soon be enrolling participants in a study of an investigational mRNA flu vaccine. Explore our currently enrolling trials to participate in ongoing clinical research and learn more.
Looking Ahead to Flu Season 2025 and Beyond
The flu will always be a seasonal challenge (at least for the foreseeable future), but with innovations like the mRNA flu vaccine, protection against the flu and other similar viruses could soon be stronger, faster, and more effective.
As we look toward flu season 2025 and beyond, today’s clinical trials are laying the groundwork to improve vaccines for the future. That said, mRNA flu vaccines are not yet available outside of research studies. For now, it’s still important to stay up to date with currently approved flu vaccines to protect yourself during this and upcoming flu seasons.
Latest Insights

What Is LDL Cholesterol? Understanding the “Bad” Cholesterol and Why It Matters

A New Path to Quit Smoking: Exploring the Next Treatment
